Introduction: Hemoglobin (Hb) Agrinio (HBA2: c.89T>C (Leu29Pro) mutation) is a rare highly unstable Hb variant resulting from a leucine to proline substitution at codon29 of the alpha2-globin gene. Eleven homozygous cases of this non-deletional alpha thalassemia were described in literature. Clinical course ranged from moderate (non-transfusion dependent anemia) to severe (transfusion dependent thalassemia). In addition, one probable case of Hb barts hydrops fetalis was also reported in a Greek series. Hb Agrinio was first described in individuals of Greek or Cypriot origins. Subsequently, some cases were reported among patients living in Spain and Macedonia, most often of Gypsy origin. We report here the clinical presentation of the 8 homozygous cases diagnosed by molecular biology analysis in France and underline the severity of the antenatal phenotype of the disease.
Methods: Cases were identified by contacting all the French laboratories involved in the molecular diagnosis of hereditary red blood cell (RBC) disorders. Three different families were retrieved, including 8 pediatric homozygous cases diagnosed by alpha globin gene sequencing between 2006 and 2020. Standardized clinical and laboratory data were obtained from treatment centers. All parents gave their written consent for molecular analysis and their authorization for participating in this study.
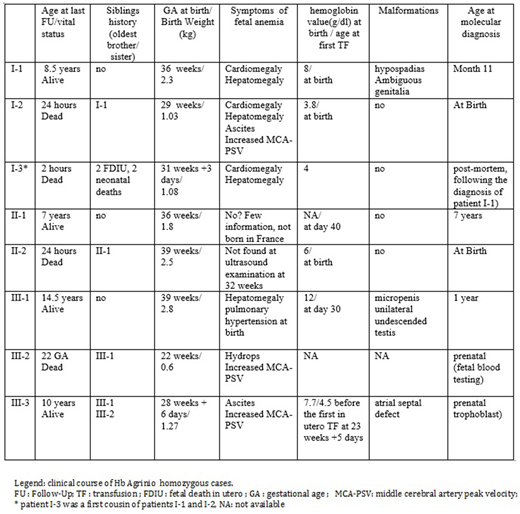
Results: Consanguinity was present in all 3 families, however no obvious link was found between the 3. Two originated from Spain (1 of gypsy origin) and 1 from Bulgaria. All patients were homozygous for the HBA2: c.89T>C mutation, without any additional beta or alpha anomalies using gene sequencing and MLPA.
Main clinical presentation findings of homozygous patients are reported in the table. Among the 8 cases, there was one in utero death at 22 weeks GA and 7 living births. Three patients, the more anemic at birth, died during the first hours of life with a clinical picture of hydrops fetalis for 2 of them. Despite a normal cerebral doppler at 32 weeks of GA, the third newborn (II2), presented at birth with a blueberry syndrome with cutaneous and hepatic extramedullary hematopoiesis, severe pulmonary arterial hypertension and Hb value of 6 g/dl, leading to neonatal death. Among the 4 survivor patients, one (III3), diagnosed in the antenatal period, benefited from in utero transfusions (TF) administered at 23 and 27 weeks because an accelerated middle cerebral artery peak systolic velocity of 52cm/s was measured. Hypospadias with ambiguous genitalia requiring surgical treatment was present in one patient and micropenis treated medically and undescended testis in a second one.
All survivors (n=4/8) are currently under regular TF and chelation therapy. TF were generally (3/4) occasional in the first years of life and then became regular (>8 TF/year) later in childhood (at 6, 6, 7 years of age respectively).
Conclusions: our national experience of homozygous Hb Agrinio cases revealed a more severe clinical course than reported in literature, with clinical presentation ranging from alpha-thalassemia major (Hb Barts hydrops fetalis) to severe non deletional HbH disease. Antenatal anemia was profound. In utero or neonatal deaths concerned half of the patients and additional ones were, even not biologically confirmed, also found in the family histories. Of note, congenital urogenital abnormalities similar to those found in the classical Hb Bart's Hydrops FetalisSyndrome were found in 2 patients. This up to now unreported severity could be explained by a closer monitoring and a more comprehensive clinical study of the cases/siblings allowing the antenatal period to be better described. The existence of aggravating genetic factor is another hypothesis and NGS analysis of genes involved in RBC disorders is planned.
In couples at risk for homozygous Hb Agrinio, early genetic counseling and prenatal diagnosis should be offered. If the pregnancy is continued, a very close fetal ultrasound monitoring is mandatory and intrauterine TFs can be administered when severe fetal anemia is detected.
Thuret:Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Investigator in clinical trials; Novartis pharma: Membership on an entity's Board of Directors or advisory committees, Other: Investigator in clinical trials; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Investigator in clinical trials; Apopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal